Home >
News>
Market News >
Clariant's injection-grade excipient VitiPure™ LEX 3350 S has been granted a CDE registration number
Home >
News>
Market News >
Clariant's injection-grade excipient VitiPure™ LEX 3350 S has been granted a CDE registration number
Clariant's injection-grade excipient VitiPure™ LEX 3350 S has been granted a CDE registration number
 Release time:2025-01-06
Release time:2025-01-06
 342
342
Clariant's pharmaceutical grade excipient VitiPure™ LEX 3350 S has been awarded the CDE registration number: F20240000562. This is another significant advancement following the successful approval of the oral grade polyethylene glycol Polyglykol™ 3350 and Polyglykol™ 4000 active pharmaceutical ingredients, as well as the Polyglykol™ 6000 excipient. This not only enriches Clariant's portfolio of polyethylene glycol products but also demonstrates Clariant's continuous contribution and long-term commitment to the pharmaceutical and healthcare industry.
Clariant is a global leader in specialty chemicals, headquartered in Muttenz, near Basel, Switzerland. Clariant's Healthcare business line is dedicated to providing high-quality product solutions for customers in the pharmaceutical and healthcare sectors, covering pharmaceutical excipients and active pharmaceutical ingredients (APIs), offering solid support for the growing demand for high-quality pharmaceutical raw and excipients.
In the European pharmaceutical industry, polyethylene glycol (Polyethylene glycol) is generally also known as Macrogol, produced by catalytic polymerization of ethylene oxide (EO) under alkaline conditions. Currently, Clariant has developed a variety of polyethylene glycol products with average molecular weights ranging from 200 to 35,000. Clariant's polyethylene glycol 3350 is one of the star products. As one of the major suppliers of polyethylene glycol (PEG), Clariant is not only able to provide diversified solutions for the pharmaceutical industry but also offer professional technical support and regulatory services in various fields, better supporting the development and promotion of pharmaceutical health products. Currently, polyethylene glycol 3350 has been widely used in the pharmaceutical industry as a stabilizer in long-acting suspension formulations. VitiPure™ LEX is an excipient developed by Clariant for high-risk products, strictly controlling microbial and bacterial endotoxins, and is particularly suitable for injectables, inhalants, suppositories, eye drops, etc.

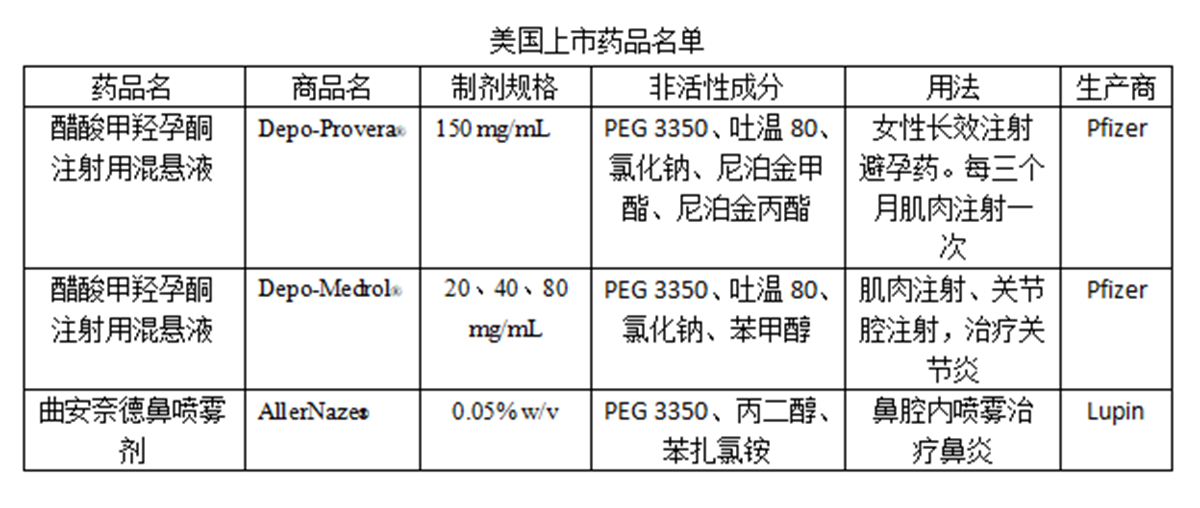 With the registration of VitiPure™LEX 3350 S excipient by the CDE, Clariant is now better able to meet the high-specification excipients needs of the pharmaceutical market.
With the registration of VitiPure™LEX 3350 S excipient by the CDE, Clariant is now better able to meet the high-specification excipients needs of the pharmaceutical market.
 Hotline:021-63534350/63534360
Hotline:021-63534350/63534360